What is a therapeutic good?
Therapeutic goods are broadly defined as products for use in humans in connection with:
- preventing, diagnosing, curing or alleviating a disease, ailment, defect or injury
- influencing, inhibiting or modifying a physiological process
- testing the susceptibility of persons to a disease or ailment
- influencing, controlling or preventing conception
- testing for pregnancy.
What is a CTN?
A CTN (or Clinical Trial Notification) is a scheme which notifies the TGA about a clinical trial using an 'unapproved' therapeutic good to be used in human research. Alongside the CTN submission, you need approval from:
- The Human Research Ethics Committee (HREC)
- The institution or organisation where the trial will be carried out (Approving Authority)
It is the responsibility of the Australian clinical trial sponsor to have all relevant approvals in place. When ACU is the trial sponsor, the responsibility to obtain the CTN is delegated to the Research Team. You must obtain the CTN before supplying the unapproved therapeutic good in the clinical trial.
If you are unsure whether you are using a therapeutic good you can find more information here.
When is a CTN required?
The use of an 'unapproved' therapeutic good must be the subject of an exemption, approval or authority under the Therapeutic Goods Act 1989. 'Unapproved' therapeutic goods refers to:
- therapeutic goods already included in the ARTG to be used in a manner not covered by the existing entry in the ARTG.
- any medicine not included in the ARTG
- any medical device not included in the ARTG
- any biological not included in the ARTG
For full definition refer to the TGA Australian Clinical Trial Handbook.
Compounded therapeutic goods (and placebos) do not appear on the ARTG and should have a CTN.
What if I am just using an unapproved drug or device as part of my data collection or evaluations rather than as the intervention?
Yes, this would still require a CTN as the therapeutic good is unapproved for the use included in your trial. All 'unapproved' therapeutic goods, not just the investigational product(s), must be the subject of an exemption, approval or authority under the Therapeutic Goods Act 1989 to be lawfully supplied in a clinical trial. For more information on what would require a CTN - refer to the TGA Clinical Trial Handbook.
- EG: repurposing a drug to dilate blood vessels for a scan where the scan is being used to assist in evaluating the efficacy of the clinical trial intervention.
- EG: using a "research" model of a blood glucose monitor to collect data, where the "research" model is not listed on the ARTG.
For devices (as per ISO14155), please be aware that the definition of clinical trial (clinical investigation) includes:
- Exploratory - A clinical investigation that might not have pre-specified primary hypotheses, and can be conducted to generate hypotheses, to be confirmed in subsequent studies.
- Confirmatory - adequately controlled clinical investigation in which the hypotheses of the primary endpoint(s) are stated before the start of the clinical investigation in the research protocol and are analysed in accordance with the protocol.
Interventional Clinical Investigation is defined as pre- or post- market investigation where the assignment of a participant to a particular medical device is described in the protocol, or diagnostic or monitoring procedure requested in the protocol are in addition to those available as normal clinical practice and burden the subject.
What else should I do?
Whenever using an "unapproved" therapeutic good, it is important to clearly advise participants in your study that the use of the drug, device or biological is unapproved and clearly explain any risks that this may entail.
This must be done for each "unapproved" product.
EG: We will be utilising a new device for measuring your blood pressure during the trial. This device is not currently approved for use to assess blood pressure. The device use involves placing a clamp on your ear which will take a small blood sample and measure the pulse in your ear and convert this to a blood pressure reading. Risks associated with using this new device include minor bruising, minor bleeding and possible infections. To mitigate these risks, we will ensure that your ear is cleaned both before and after the clamp is applied.
Applying for a new Clinical Trial Notification
New Clinical Trial Notifications (CTN) to the TGA should be completed by the research team. Contact res.ethics@acu.edu.au if you do not already have an account. A user account will be set up on your behalf. Once this is done, you will receive an email from the TGA containing a link to choose a password. Further assistance can also be found at the TGA User Guide.
- Log in to the TGA Business Services Portal using your log in details.
- Click on 'Applications' then select 'Clinical Trial Notification'
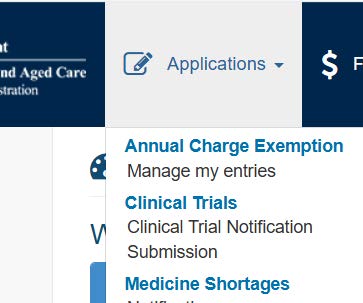
- Click on 'Trial Details' and proceed to complete the form that opens. Remember to save each completed section so you do not lose information already entered.
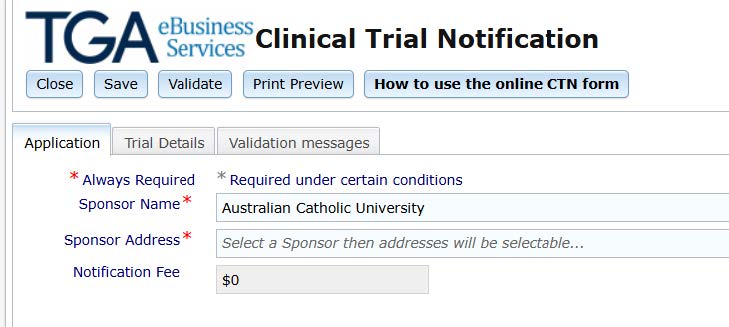
- Once completed click the 'Validate' button and it will check for errors. The status will change from 'Draft' to 'Validated' once filled in completely.
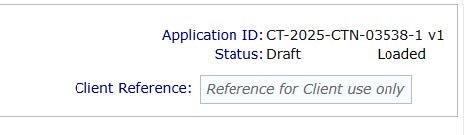
- Once validation has taken place a 'SUBMIT' button will appear on the same tab line.
Once a submission has been approved it will move to the repository and is considered finalised.
Modifying an existing CTN
If you need to modify your existing CTN:
- Log in to the TGA Business Services Portal using your log in details.
- From the home page, click on 'View submissions' under 'My work'.
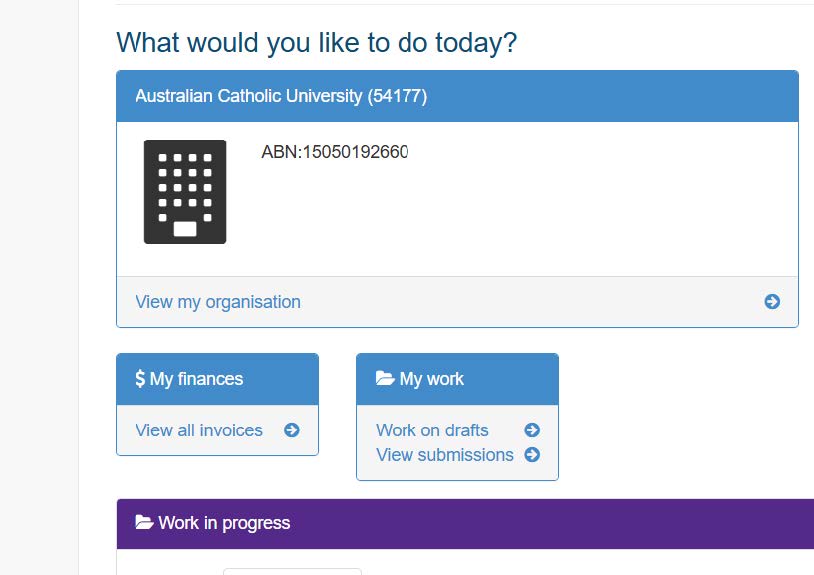
- Select 'View lodged submissions' to locate your trial in the repository.
- Once you have located your clinical trial, click on the down arrow next to the ID number and select 'Vary'.
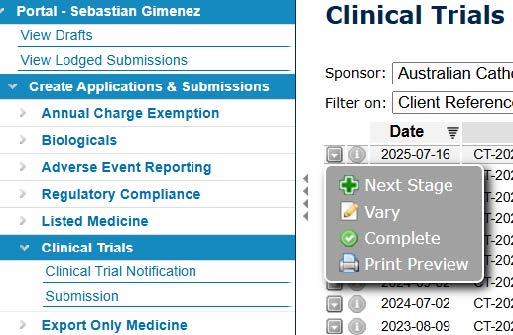
- Make the updates to the CTN as necessary. You can conduct a print preview before you accept and submit.
Once the change has been approved the trial will return back to the repository.







